Templated Enzymatic Synthesis of Cyclodextrins: From Redox-Responsive Systems to Preparative Scale Synthesis
Cyclodextrins (CDs) are water-soluble macrocyclic carbohydrates, formed from glucose units linked in a ring. Tonnes of cyclodextrins containing 6, 7 and 8 glucose units are produced annually for applications in the pharmaceutical, food and cosmetic industries, where they are used as container molecules that can encapsulate hydrophobic guests. CDs are produced by the enzymatic degradation of starch using the enzyme, cyclodextrin glucanotransferase (CGTase). Cyclodextrins consisting of more than 8 glucose units are called large-ring cyclodextrins (LRCDs) and are extremely rare. A method for producing LRCDs in large amounts has not previously been developed, and, therefore, they have remained relatively unexplored.
This thesis shows how CGTase can be used to generate dynamic combinatorial libraries (DCLs) of interconverting cyclodextrins in which the distribution of cyclodextrins formed can be controlled by adding different template (guest) molecules that bind to and stabilise specific cyclodextrins (Figure 1). The first part of this thesis presents the use of redox-responsive ‘breakable’ templates and shows that the production of different CDs can be controlled depending on whether or not the template is cleaved. Secondly, a chromatography-free, high-yielding, scalable method for the enzymatic synthesis of a large-ring cyclodextrin with 9 glucose units (d-CD) is presented, in which an icosahedral dodecaborate anion is used as the template. Furthermore, using an engineered CGTase, the large-ring cyclodextrin with 10 glucose units (e-CD) could be produced with unprecedented selectivity. Finally, insight into the binding properties of the newly accessible d-CD host is presented. Binding studies using NMR spectroscopy revealed a strong and size-dependent affinity for different dye molecules.
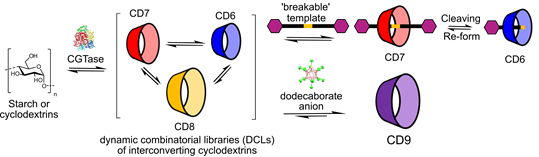
Figure 1. Illustration of templated enzymatic synthesis of cyclodextrins (CDs) using enzyme-mediated dynamic combinatorial chemistry.
Principal Supervisor:
Associate Professor Sophie Beeren, DTU Chemistry
Co-supervisor:
Senior Researcher Dennis Larsen, DTU Chemistry
Examiners:
Associate Professor Charlotte Held Gotfredsen, DTU Chemistry
Associate Professor Fabien Cougnon, University of Jyväskylä, Finland
Professor Kim Lambertsen Larsen, Aalborg University
Chairperson:
Professor Jens Øllgaard Duus, DTU Chemistry