Manganese-Catalyzed Coupling Reactions
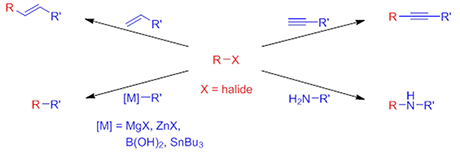
The cross coupling reaction has been one of the most important discoveries in organic chemistry where a carbon-carbon bond or a carbon-nitrogen bond in formed by combining two different fragments R and R’. The former is bound to a halide while the latter is connected to a metal, an alkene, an alkyne or an amine. The coupling has had a tremendous impact on the pharmaceutical industry where it accounts for about 10% of all reactions used in the synthesis of small molecule pharmaceuticals. However, the reaction suffers from one major drawback: the metal palladium is used as the catalyst.
Palladium does not occur naturally in the human body and all palladium compounds are considered toxic. The price of palladium is about $20,000/kg and the annual production is limited. On the contrary, manganese is sold for about $3/kg and is relatively non-toxic since it is an essential element in all living organisms. As a result, manganese catalysts would be an extremely valuable alternative to the palladium counterparts in the cross coupling reaction.
The development of the manganese-catalyzed cross coupling reaction takes advantage of a dual approach where catalyst screening and development are pursued together with experimental and computational mechanistic studies at the single atom level. In this way, catalyst optimization can be guided by in-depth knowledge of the elementary steps in the catalytic processes. Successful manganese catalysts will be evaluated on a variety of substrates to explore the scope and limitations of the new cross coupling protocols. The project is funded by the Danish Council for Independent Research – Technology and Production Sciences and carried out in collaboration with Peter Fristrup and Per-Ola Norrby.
Project participants
Professor Robert Madsen
Technical University of Denmark
rm@kemi.dtu.dk
Associate Professor Peter Fristrup
Technical University of Denmark
pf@kemi.dtu.dk
Professor Per-Ola Norrby
University of Gothenburg
pon@chem.gu.se
Glycoside synthesis
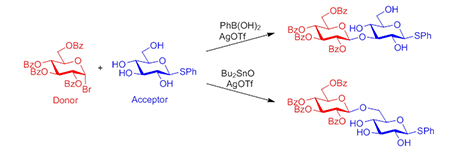
The most important linkage in carbohydrates is the glycosidic bond which is prepared by a glycosylation reaction where a glycosyl donor is activated by a promoter and coupled to a partially protected acceptor. Usually, oligosaccharides are assembled by a series of stepwise glycosylations where protected donors and acceptors are coupled followed by removal of the protecting groups. Although the protecting groups are important for directing the glycosylations, their use also adds many additional steps to the synthesis of a target molecule.
The group is involved in two strategies to diminish the use of protecting groups in oligosaccharide synthesis. In the first, several consecutive glycosylations are carried out in the same pot by using chemoselective couplings with thioglycosides. Two thioglycosides are selectively connected in such a way that one serves as the donor and the other as the acceptor. The resulting disaccharide is also a thioglycoside and can be coupled again in the same reaction. By using these chemoselective couplings two or more glycosylations can be performed in the same pot which saves several steps in the preparation of a target oligosaccharide.
In the second strategy, regioselective glycosylations are performed with unprotected glycosyl acceptors. Instead of using protecting groups to distinguish between the hydroxy groups, a directing agent is mixed with the acceptor followed by a glycosylation with a glycosyl donor. The directing agent binds to the hydroxy groups in the acceptor and either enhances or diminishes their reactivity. In this way, one hydroxy group becomes significantly more reactive than the others making a regioselective glycosylation possible.
SET4Future