Ultraviolet Raman spectrometry of oil mixtures, biological samples and catalysts; an optical method for characterization of fluorescing materials.
Department of Chemistry DTU has deep UV-Raman spectroscopy as a multi disciplinary research field

Fig.1 Deep UV Argon-ion laser (Lexel 95-SHG™ )

Fig.2 Deep UV Raman instrument (Renishaw plc, UK)
In Raman spectroscopy fluorescence (that is emission of unwanted light) is a persistent problem. Surprisingly, it has been discovered that a way to avoid this fluorescence is to excite the Raman spectra with deep ultraviolet (DUV) light. “Fluorescence does not exist if the exciting light has a wavelength shorter than 260 nm” [1]. The DTU project has accordingly been started to get experience with the use of DUV-laser light in Raman spectroscopy to characterize samples that with visible light would be strongly fluorescing. This method has only - over the many years since 1984 - been very sparsely investigated. We foresee many applications of results of the DUV Raman method. A main focus is on characterization of food and oil products. The DUV Raman technique - when implemented – will help oil characterization and identification of waste products found at sea and shores. Small amounts of polycyclic aromatic hydrocarbons (PAHs) and sulfur-containing compounds contained in oil products should be detectable (on a ppm to ppb scale or less).
A major aim in the project has dealt with the implementation of this not so easy kind of deep UV spectroscopy in practice. With regard to DUV light sources, our laboratory is p.t. equipped with two continuous wave (CW) lasers. The first one is based on the second harmonic generation (SHG) from an Ar-ion laser. The second one is a 4th harmonic line generated from a Nd:YVO4 laser.
1. A DUV Ar-ion laser, (Lexel 95-SHG™ laser, see Fig. 1).
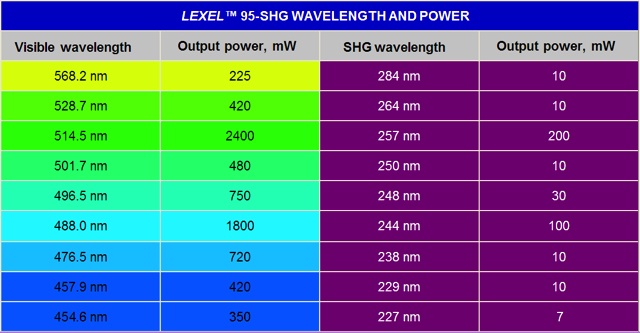
Ar-ion lasers emit at several wavelengths through the visible and ultraviolet spectrum. The most useful lines are those occurring at 351 nm, 457.9 nm, 488.0 nm, 496.5 nm, 514.5 nm. The strongest laser lines are those at 514.5 nm and 488.0 nm. The Ar-laser can be forced to run single mode and emit at single wavelengths by use of an intracavity etalon and a prism. Normally, the Ar-ion laser is a continuous wave (CW) laser. To archive effective SHG, the intracavity frequency doubling techniques [3, 4] can be used, giving laser emission at wavelengths of 229 nm, 244 nm, 248 nm and 257 nm. Our laser is made by Cambridge Lasers Laboratories, INC (USA). The Lexel 95-SHG™ laser is using the intra-cavity technique [3] to produce SHG DUV-laser light in nonlinear crystals of Beta Barium Borate (BaB2O4), also called BBO. The emission wavelengths and typically output powers are shown in Table 1. Each DUV-line requires its own BBO crystal with differently-cut angle and orientation optimized for that particular wavelength. Presently, our laboratory is equipped with the BBO crystals for 257 nm, 244 nm and 229 nm. The crystal for 229 nm generation can also be used for 227.3 nm generation.
Table 1. Argon-ion laser lines, wavelengths and power levels.
2. A solid-state 266 nm laser (CRYLAS FQCW 266-50 laser, see Fig. 3)
The FQCW 266-50 laser is made by the German company CRYLAS GmbH. This highly stable laser emits a CW DUV laser beam with a fixed wavelength of 266 nm. The light is the 4th harmonic generation from a strong Nd:YVO4 laser. This Nd:YVO4 laser emits at 1064 nm with single longitudinal mode operation (so-called single frequency). The fundamental light source of the FQCW 266 -50 laser is a diode pumped solid state laser (a DPSS laser) pumped by the 808 nm laser diodes. The 4th harmonic generation from this is obtained by two consecutive frequency doubling stages. Each stage is a resonant enhancement cavity (also called external power-enhancement cavity) equipped with a respective second harmonic generation (SHG) nonlinear crystal. In the first stage LiB3O5 is used (also called LBO) and for the second stage BBO is used. For detailed optical properties we can recommend the homepage of the manufacturer: www.crylas.de/products/cw_laser.html

Fig. 3: The 266 nm solid-state laser, CRYLAS FQCW 266-50.
We have several Raman systems installed in the Lab. The UV Raman system is a Renishaw™ instrument (Fig. 2), equipped with UV-enhanced Suprasil™ quartz optics, a microscope, a highly sensitive CCD detector, and the necessary accessories. The setup of our system is shown in Fig. 4.
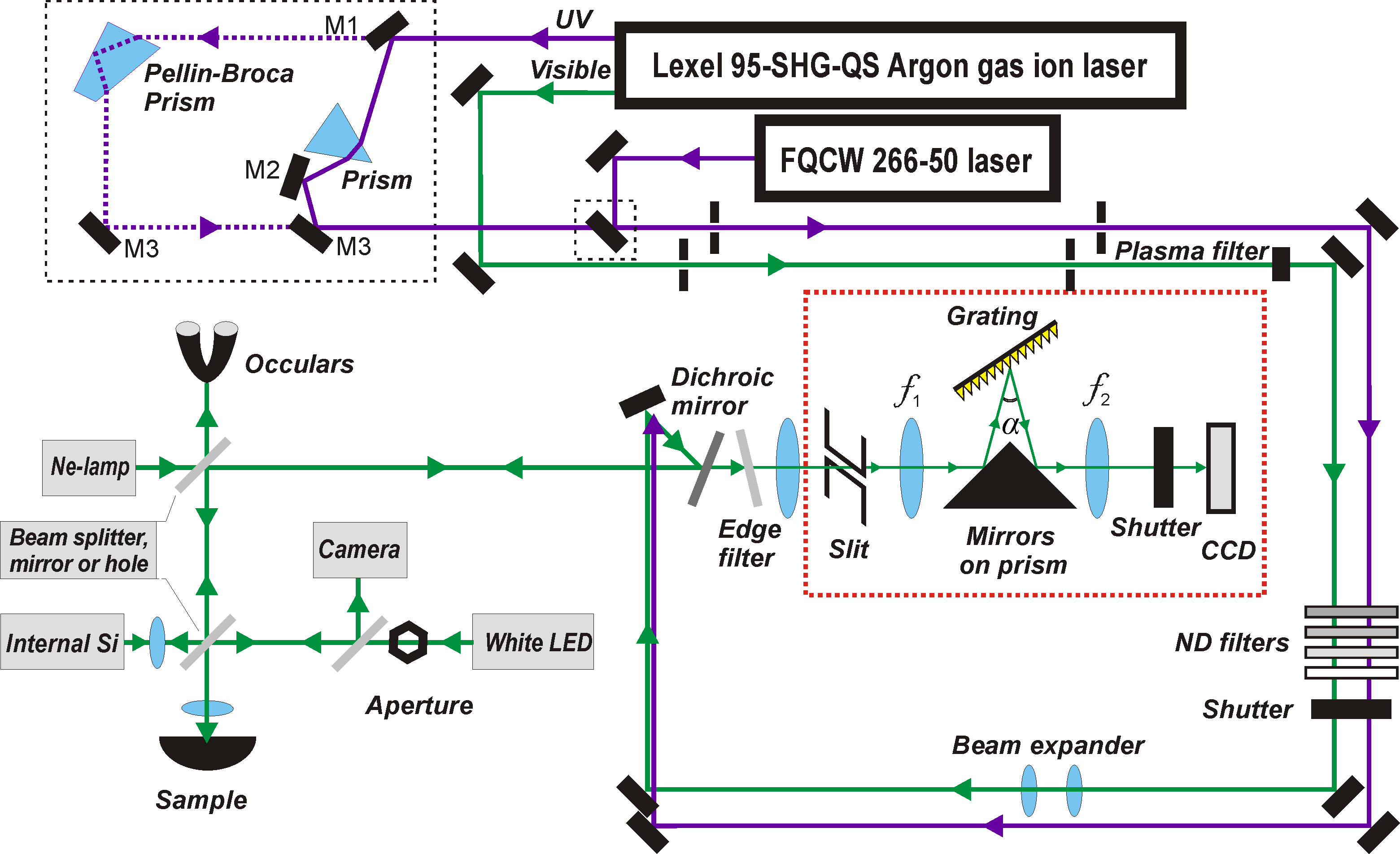
Fig. 4: The UV Raman Setup in our laboratory.
An important future aspect of the DUV-Raman method is application of optical fibres to totally encapsulate the UV radiation, thus avoiding any risk of damage to persons. Optical hollow micro-structured “photonic crystal” quartz fibres with minute air-holes may be used, guiding light to pipettes with sample chemicals and solutions (e.g. series of oil/gasoline/methanol mixtures) that one wants to analyze.
Applications
Gasoline analysis
Previously an attempt to develop on-line gasoline Raman analysis has been tried with exciting light from a 514.5 nm laser [5]. The measurements showed strong fluorescence. Thus, analysis was difficult. We used the 229 and 244 nm laser lines as the exciting sources, and got Raman spectra with clear bands and weak fluorescence interferences (Fig. 5 and 6). Gasoline is a mixture with hundreds compounds. Spectrum interpretation is a challenge: The bands seen are mainly from compounds which have resonance enhancements. We found that the spectra of gasoline by use of 229 nm laser excitation are dominated by e.g. the naphthalene component due to its resonance enhancement as shown in Fig. 5. Similarly, the spectra of gasoline by use of 244 nm laser excitation are mainly contributed by naphthalene and toluene as Fig. 6 shown. Two intensity bands of 1004 and 1381 cm-1 can be assigned to the monocyclic and bicyclic ring breathing modes in Toluene and Naphthalene respectively as shown on Fig.7.
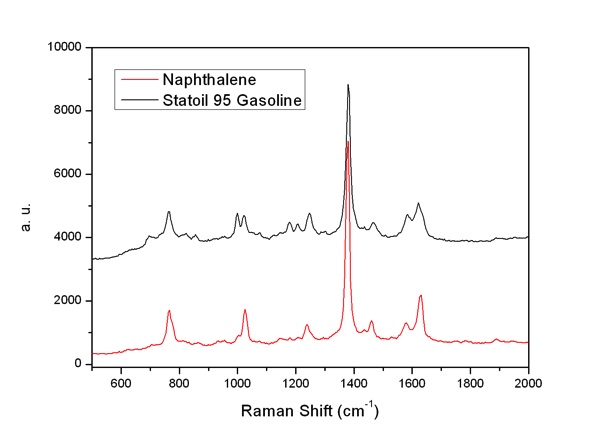
Fig. 5: Raman spectra of Statoil 95 Gasoline and Naphthalene, excited with the 229 nm laser.
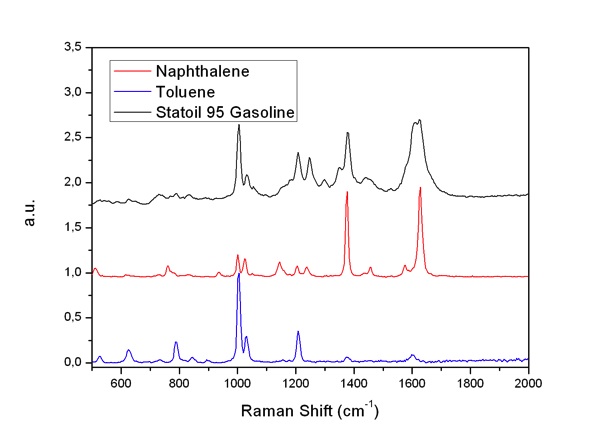
Fig. 6: Raman spectra of Statoil 95 Gasoline, Toluene and Naphthalene, respectively. Excited with 244 nm laser
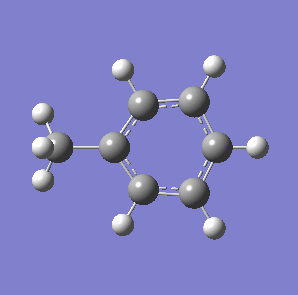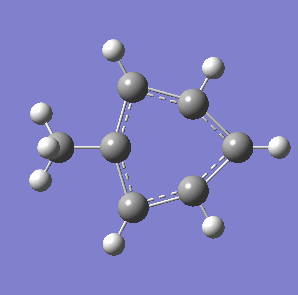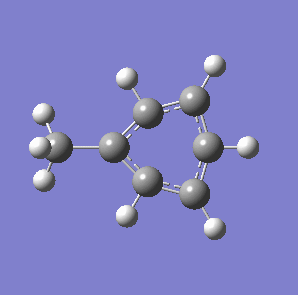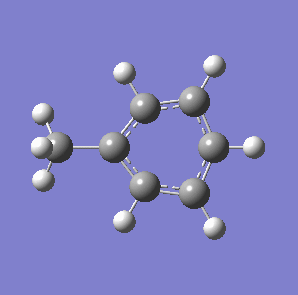
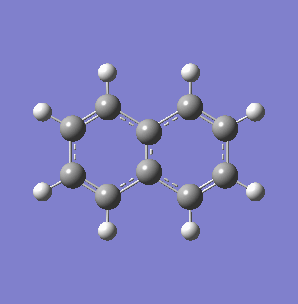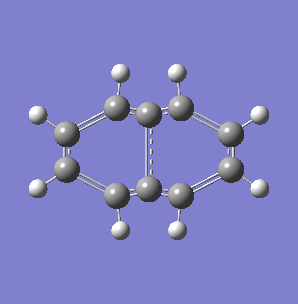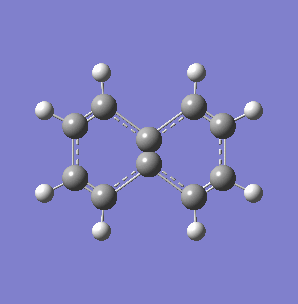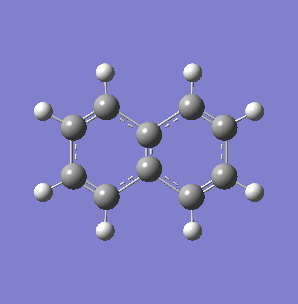
Fig. 7: (left) The monocyclic ring breathing mode in Toluene at 1004 cm-1; (right) the bicyclic ring breathing mode in naphthalene at 1381 cm-1. The calculated vibrational modes and Raman active bands were obtained by use of the Gaussian 03W software package. The optimisation of the molecular structure were performed. The structure of the Melamine molecule and its vibrations were obtained iteratively, in three subsequent calculations. Initially the geometry was roughly optimized using the semi-empirical PM3 type procedure, followed next by the ab initio Hartree-Fock/DFT method with 3rd order restricted Becke-Lee-Yang-Parr procedure (RB3LYP), at first with the 3-21G basis sets. Then the energy minimum structure and the vibrational modes were calculated by using the 6-31+G basis sets.
Food additives: Sudan I

The red dye Sudan I is considered to be toxic and carcinogenic. In recent years, several European control authorities have discovered the presence of Sudan I in series of imported food items, such as red canned chili fruits [6]. The recently applied methods for determination of Sudan I give detection limits on the order of around 0.5 ppm [7]. No known threshold limit seems to exist with respect to long-term health risks, and therefore there is a need for further development of the detection methods.
a) b)
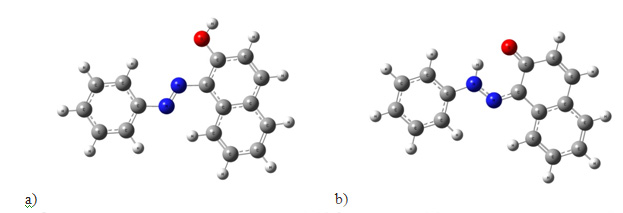
Scheme 1. The tautomeric inversion of Sudan I: (a) the azo form and (b) the hydrazo form. The equilibrium is shifted far to the right. The structure of azo dyes has been intensely discussed in the chemical literature. The azo dyes formally contain the azo group –N=N–, but are in most cases partly or completely transformed into the tautomeric hydrazo form (Scheme 1, right). According to our studies with the Gaussian 03W software package, the azo forms gave poor resemblance to the experiments. In contrast to this, the calculated spectrum for the hydrazo form showed good resemblance to the experimental spectrum [8].
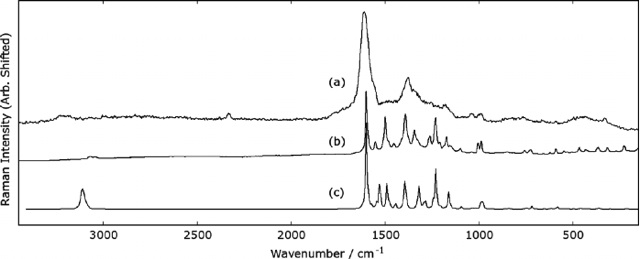
Fig. 8: Raman spectra of solid Sudan I obtained with (a) 244 nm UV light and (b) 1064 nm IR light. Useful spectra could not be obtained with green or blue light. The calculated spectrum is shown as (c). The band at 2331 cm-1 in (a) is due to N2 gas.
Size-effect of germanium (Ge) nanocrystals
Recently, zero-dimensional nanocrystals have been intensively studied in theoretical and experimental research, due to potential applications as light emitters, non-volatile optical memories and enhanced 3rd order optical nonlinear effects, etc. Among these nanocrystals, the high-efficiency germanium light emitter application is receiving much attention because Ge has a 0.80 eV direct bandgap, only slightly larger (by 0.136 eV) than its indirect bandgap (0.664 eV). This puts Ge emission at high efficiency, within the 3rd optical communication window (~1520-1620 nm). Bulk Ge emits with 10% efficiency at a direct bandgap wavelength of 1550 nm when optimized doping and is applied. In Ge nanocrystals usually the emission wavelength is shifted to shorter wavelengths because of the quantum confinement effect (QCE). Therefore, Ge nanocrystals with combined effects of QCE, doping and strain optimization should be very promising high-efficiency light emitters, covering the entire visible and IR wavelength ranges.
In this project we studied different sizes of Ge nanocrystals embedded in a SiO2 matrix formed by plasma enhanced chemical vapor deposition (PECVD), and analyzed the nanocrystals by transmission electron microscopy (TEM), see Fig. 9 below.
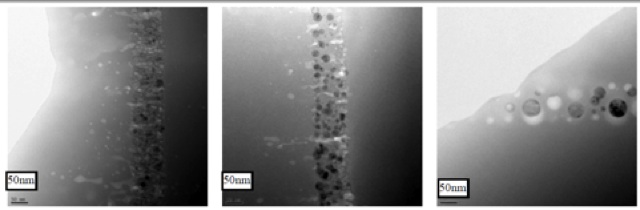
Fig. 9: TEM cross-sectional images of samples (from left to right): 0126, 0269 and 0194.
Samples were prepared by standard clean-room techniques on 4 inch (100) oriented Si substrates. First, one layer of Ge doped SiO2 was deposited. Then a SiGe alloy layer was deposited on top of thts SiO2 layer. The ratios of the Si to Ge were tuned from 1:1 to 1:0.5 and 1:0.25 for samples 0194, 0269 and 0126 respectively. Thirdly, one identical layer of Ge doped SiO2 was deposited on the SiGe alloy layer. By repeating the deposition of alternate layers of SiGe alloy and Ge doped SiO2, multilayered structures were readily made. Samples were annealed at 1100°C in N2 for 4 hours after deposition. More details of the fabrication parameters are found in [9]. Samples 0126, 0269 and 0194 had 3-layer structures. The Ge nanocrystals were found to give Raman peaks at about 295-300 cm-1, shifting linearly with the excitation power density. Sample 0351 had a 5-layer structure with 1:1 Si/Ge ratio, made in order to increase the interaction with the light. A size effect was demonstrated within these Ge nanocystals (black dots in Fig. 9) after excluding any thermal effect as having effect on the properties, see Fig. 10 and [10,11].
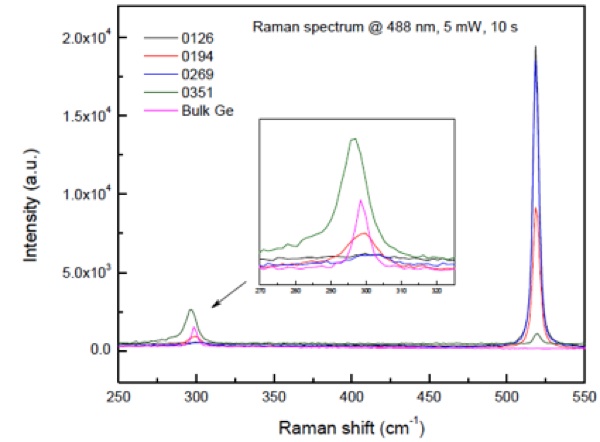
Fig.10: Raman spectra of samples 0126, 0269, 0194, 0351 and a reference bulk Ge wafer. The inset is a zoom-in on the main peak of the Germanium lattice [10,11].
Solid state technology
White light-emitting diodes (LEDs) are promising candidates for the illumination market to replace conventional incandescent lamps as energy-saving and environment friendly light sources. One common method to realize white LEDs is using a UV or blue LED whose light is partially or fully used to optically excite wavelength converters. Therefore, high-efficiency wavelength converters with long lifetime are indispensable. SiC is a well established substrate material for nitride growth and has excellent thermal conductivity. Nitrogen (N) and boron (B) doped 6H-silicon carbide (SiC) has been proven to be highly efficient wavelength converters. Furthermore, combined donor-acceptor-pair (DAP) band luminescence from N-B and nitrogen-aluminium (N-Al) doped 6H-SiC can cover most of the visible spectral range [12-14].
In this connection characterization of nitrogen and boron doped siliconcarbide by UV Raman spectroscopy is of interest. Hence, doped epilayers grown on low off-axis 6H-SiC substrates have been studied in our laboratory [13, 14]. Raman spectra of longitudinal optical phonon-plasmon coupled (LOPC) modes in the samples are shown in Fig. 11. It was found that N-B doped SiC is a good wavelength converter in white LEDs applications. A dopant concentration difference larger than 4x1018 cm-3 is favorable to achieve intense photoluminescence.

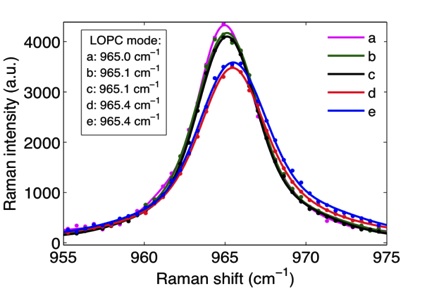
Fig. 11: Raman backscattering spectra of 6H-SiC samples with different dopant concentrations showing longitudinal optical phonon-plasmon coupled (LOPC) modes (inset: positions of the LOPC modes of doped SiC samples).
The mechanisms of Raman shifts in p- and n-type samples are different. The LOPC mode would broaden, lower its intensity, and shift toward higher wavenumbers with increasing free carrier concentrations. Usually, it is more sensitive to the amount of free electrons than to free holes. In p-type samples, very few acceptor states are ionized due to the large ionization energy, and the Raman shift is mainly contributed to the atomic size effect [14]. B atoms usually occupy Si lattice positions in SiC. The inter-atomic distance of a Si-C bond is longer than that of a B-C bond due to the smaller atomic radius of B. The biaxial tensile stress will be released which results in a decrease of the phonon oscillation frequency. So the LOPC mode shifts toward smaller wavenumbers with higher B concentrations. In n-type samples, the predominant mechanism causing the Raman shift of the LOPC mode is the free carrier (electron) concentration. Although no obvious peak shift has been observed between sample d and e due to a relatively small concentration difference, the peak intensity of the LOPC mode decreases as expected when the free electron concentration increases from sample e to d. Furthermore, one can see from Fig. 11 that the LOPC modes of n-type samples occur at significantly higher wavenumbers than the p-type ones [14].
Melamine
Melamine, C3H6N6, 2,4,6-triamino-1,3,5-triazine, CAS Reg. no. 108-78-1, is a nitrogen-rich chemical, commonly used e.g. as main raw material of melamine resin, a thermosetting plastic. Recently it has received considerable attention due to numerous incidents of food contamination with this substance [15-.17]. In these incidents, melamine was deliberately added to gluten, chicken feed, pet foods, milk etc. to elevate the measured protein content because of melamine's high nitrogen content.
As a potential method with advantages of being simple, quick, cost-effective and sensitive, Raman spectroscopy has been intensively applied to melamine detection in food systems in recent years. Most studies have been focused on the surface enhanced (SERS) techniques because of the high sensitivity properties of SERS. About a 10 ppb detection limitation has been reached [18]. The traditional Raman spectrometry - excited by 785 nm infrared laser light - is also reported to achieve a successful detection of melamine, although the sensitivity was much lower; the detection limit being about 1% in powdered milk samples [19]. However, DUV Raman spectrometry applied to melamine detection has not been reported, and we found that it would be of interest to try it. In our study it is shown that DUV Raman spectrometry could avoid the fluorescence interference and improve the detection sensitivity for direct melamine detection in liquid milk. It could realize a true quick detection in seconds without sample preparation.
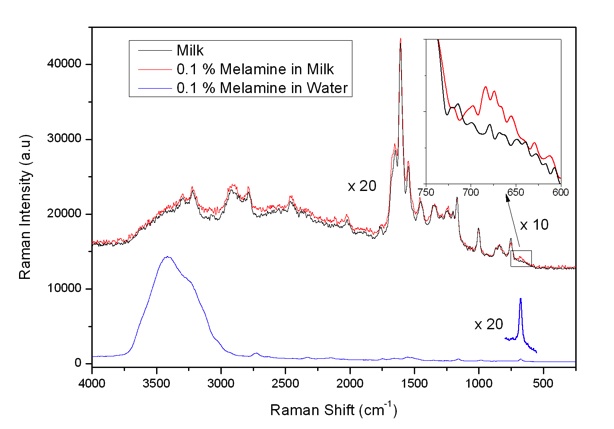
Fig. 12: Raman spectra of milk, 0.1 % melamine in water and Milk by use of 229 nm (10 mW) excitation.

Fig. 13: C3 Triangle Breathing mode coupled to NH2-scissoring (667 cm-1 on measurement, 682.0 cm-1 on calculation). Calculation methods were as given in Fig.7.
UV Raman spectroscopy resolution techniques
A work has been done on determining the spectral resolution of Raman spectrometers, comparing the resolution of instruments used in visible and UV Raman spectrometry [20],[21]. It is often important to be able to determine the resolution in the actual setup. A new method has been developed making it possible to entirely express the spectral resolution by means of principal factors and one equation. The expression has been verified by measuring the band width of the 1332.4 cm-1 diamond Raman fundamental band, excited with quite different wavelengths: 257.3 nm (ultraviolet) and 514.5 nm (visible), see Fig. 14. Verification has also been done with the low pressure mercury line at 265.2042 nm (ultraviolet). The expression is based on the approximation of a Gaussian shape of the bands. Finally, a useful method to find the true Raman band width is provided. An essential feature of the new method is the demonstration of a way to compensate for the non-ideality of the instruments (diffractions, aberrations, etc.). Another finding is that the spectral resolution exhibits a significant change along the Raman shift axis. Although not new, this feature has been often overlooked in many contemporary works reporting Raman spectra. Also, the static multichannel recording and synchronous scanning (extended) recording mode differ with respect to their resolution properties, see Fig. 15.
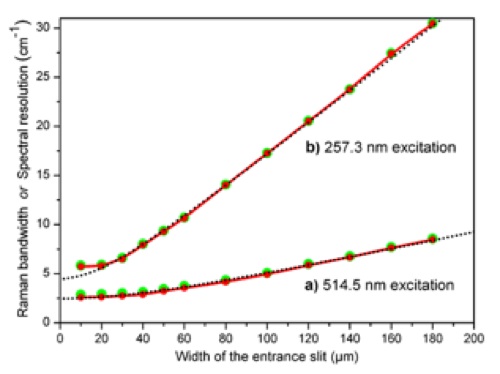
Fig. 14: Raman spectral resolution modeling. The wide green points are the measurements of Raman bandwidth ΔωM of diamond for various slit widths. The narrow red points, connected by red curves, are the experimental spectral resolution data Δω obtained by means of the new model equation [20, 21]. The black dotted curve is the theoretical spectral resolution results (spectral resolution) based on our first principle equations [20, 21]. a) Green light source. b) Ultraviolet light source.
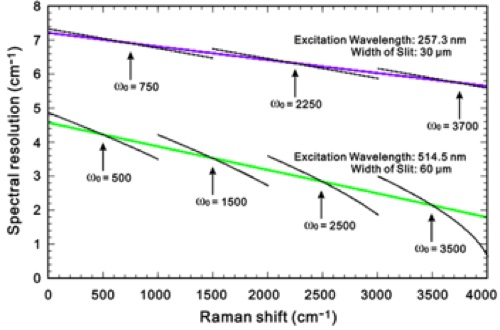
Fig. 15: The observed dependence of the spectral resolution along the Raman shift axis [20, 21]. The green and violet lines show the theoretical results calculated by use of our equation for only the central part of the CCD (in extended scanning mode using only few pixels). The black lines show the spectral resolution increments in four spectrograms and three spectrograms, for 514.5 nm and 257.3 nm laser excitation, respectively, obtained in static mode recordings, using many pixels and settings of the central pixel of the CCD at Raman shift wavenumbers of ω0 = 500, 1500, 2500 and 3500 cm-1 for 514.5 nm excitation, and ωo = 750, 2250 and 3750 cm-1 for 257.3 nm excitation, respectively.
General on UV
The UV Raman Spectroscopy project is an interdisciplinary collaboration between researchers at the Technical University of Denmark (DTU) and other universities. We seek to combine competences to construct a versatile technology platform with the vision of achieving a technique, which is applicable to analysis of any sample.
The UV Raman project was sponsored by The Danish Research Council for Technology and Production Sciences through the grant no. 274-08-0168. We thank Jakob Thyr from K-Analys, Sweden for help during the project.
REFERENCES:
[1] S. A. Asher & C. R. Johnson, “Raman spectroscopy of a coal liquid shows that fluorescence interference is minimized with ultraviolet excitation”, Science, 225, 311 (1984).
[2] solartii.com/laser/cf131a
[3] http://www.rp-photonics.com/intracavity_frequency_doubling.html
[4] http://www.rp-photonics.com/enhancement_cavities.html
[5] S. Brunsgaard Hansen, R.W. Berg and E.H. Stenby, ‘‘Determination of methyl tertiary butyl ether (MTBE) in gasoline by Raman spectroscopy’’, Asian Chem. Letters 2000, 4, 65.
[6] V. Cornet, Y. Govaert, G. Moens, J.V. Loco, J.J. Degroodt, J. Agric. Food Chem. 2006, 54, 539.
[7] H. Buchardt, Dan. Kemi 2007, 88, 28.
[8] A.J. Kunov-Kruse, S.B. Kristensen, Chuan Liu and R.W. Berg,‘‘Experimental and ab initio DFT calcd. Raman spectrum of Sudan I, a red dye’’. J. Raman Spectrosc. 2011, 42, 1470-1478.
[9] H. Ou, T.P. Rørdam, K. Rottwitt, F. Grumsen, A. Horsewell, R.W. Berg, & P. Shi, “Ge nano-clusters in PECVD-deposited glass caused only by heat treatment” Appl. Phys. B, 91, 177-181 (2008).
[10] H. Ou, Y. Ou, Chuan Liu, R.W. Berg and K. Rottwitt: “Formation and Characterization of varied size germanium nanocrystals by Electron Microscopy, Raman spectroscopy and Photoluminescence”, Optical Materials Express (Optical Society of America), 2011, Vol.1, Issue 4, pp. 643-651. (ISSN: 2159-3930).
[11] H. Ou, Y. Ou, Chuan Liu, R. W. Berg and K. Rottwitt, “Size-effect of germanium nanocrystals”, part of: Conference proceedings to CLEO:2011 Laser Science to Photonic Applications, Optical Society of America, Baltimore Convention Center, Maryland USA, 1-6 May, 2011. Full conference paper publ. in proceedings/book (3 pages).
[12] Y. Ou, D. D. Corell, C. Dam-Hansen, P. M. Petersen, H. Ou, Antireflective sub-wavelength structures for improvement of the extraction efficiency and color rendering index of monolithic white light-emitting diode, Optics Express (Optical Society of America), vol. 19 (S2), pp. A166-A172 (2011). (ISSN: 1094-4087) (DOI: 10.1364/OE.19.00A166) (paperid: We P-58).
[13] Y. Ou, V. Jokubavicius, Chuan Liu, R.W. Berg, M. Linnarsson, S. Kamiyama, Z. Lu, R. Yakimova, M. Syväjärvi, and H. Ou: “Photoluminescence and Raman spectroscopy characterization of boron- and nitrogen-doped 6H silicon carbide”, Extended Abstract to the 2011 International Conference on Silicon Carbide and Related Materials (ICSCRM 2011), September 11 – 16, 2011, Cleveland, Ohio, USA (5 pages).
[14] Y. Ou, V. Jokubavicius, S. Kamiyama, C. Liu, R. W. Berg, M. Linnarsson, R. Yakimova, M. Syväjärvi, and H. Ou, Donor-acceptor-pair emission in N-B doped fluorescent SiC, Optical Materials Express, Vol. 1, Iss. 8, pp. 1439–1446 (2011).
[15] Food and Drug Administration (USA), (2007), Interim melamine and analogues safety/risk assessment. Available from: www.cfscan.fda.gov/~dms/melamra. Accessed Feb 1, 2008.
[16] Chan EYY, Griffiths SM, Chen CW, “Public-Health Risks of Melamine in Milk Products”, (2008), Lancent 372: pp. 1444-1445.
[17] Pliny, “Melamine tainted milk re-emerges in northwest China plant”, Xinhua, news.xinhuanet.com/english2010/china/2010-07/09/c_13392414.htm. Retrieved 2010-07-09.
[18] Qiu Chao, Maingi, T. Alfred, Jiang Chaoyang, “Surface-enhanced Raman scattering detection of melamine via silver nanostructures, Abstracts of Papers, 241st ACS National Meeting & Exposition”, Anaheim, CA, United States, March 27-31, 2011.
[19] Okazaki Shigetoshi, Hiramatsu Mitsuo, Gonmori Kunio, Suzuki Osamu, Tu Anthony T., “Rapid nondestructive screening for melamine in dried milk by Raman spectroscopy”, Forensic Toxicology 27(2), (2009), pp. 94-97.
[20] Chuan Liu and R. W. Berg: “Determining the Spectral Resolution of a Charge-Coupled Device (CCD) Raman Instrument”, Appl. Spectrosc. 66(9) 2012, 1034-1043.
[21] Chuan Liu, Ph. D. Thesis, Implementation of Deep Ultraviolet Raman Spectroscopy, DTU Chemistry, 31. Dec. 2011, Printed at DTU March 2012.
[22] R. W. Berg, I. Shim, P. C. White and S. Abdali, “ROA and Raman Spectra of Amphetamine Species – Quantum Chemical Model Calculations and Experiments, America Journal of Analytical Chemistry, AJAC 2012, 3, 410-421.
[23] Chuan Liu and R. W. Berg, “Non-linearity in Intensity versus Concentration Dependence for the Deep UV Resonance Raman Spectra of Toluene and Heptane”, Appl. Spectrosc. Reviews, 48(5) (2012) 425-437.
[24] A. G. Kalampounias, G. Tsilomelekis, R.W. Berg and S. Boghosian, Molybdenum (VI) Oxosulfato Complexes in MoO3–K2S2O7–K2SO4 Molten Mixtures: Stoichiometry, Vibrational Properties and Molecular Structures, J. Phys. Chem. A 116 (2012) 8861-8872.
[25]I. Sádaba, Y. Y. Gorbanev, S. Kegnæs, S. S. R. Putluru, R. W. Berg, A. Riisager, Catalytic performance of zeolite-supported vanadia in the aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran, ChemCatChem 2013, 5, 284-293.
[26] R.W. Berg, Investigation of L(+)-Ascorbic Acid with Raman Spectroscopy in Visible and UV Light, Applied Spectroscopy Reviews, 50:3, 193-239, 2014. DOI: 10.1080/05704928.2014.952431.
For more information please contact
Associate Professor, Ph.D. Rolf W. Berg (rwb@kemi.dtu.dk )
Phone +45 4525 2512 or +45 4050 4191